By Jim Riggs1
Background
Approximately 40,000 distillation columns are operated in the U.S. chemical process industries and they comprise 95% of the separation processes for these industries. Because distillation operation directly affects product quality, process production rates and utility usage, the economic importance of distillation control is clear. Distillation control is a challenging problem because of the following factors:
▪ Process nonlinearity
▪ Multivariable coupling
▪ Severe disturbances
▪ Nonstationary behavior
Distillation columns exhibit static nonlinearity because impurity levels asymptotically approach zero. The impurity level in the overhead product is the concentration of the heavy key, and the impurity level in the bottoms product is the concentration of the light key. Nonlinear dynamics, i.e., variations in time constants with the size and direction of an input change, and static nonlinearity are much more pronounced for columns that produce high-purity products, e.g., columns that have impurity levels less than 1%.
Coupling is significant when the composition of both overhead and bottoms products are being controlled. Columns are affected by a variety of disturbances, particularly feed composition and flow upsets. Nonstationary behavior stems from changes in tray efficiencies caused by entertainment or fouling.
Improved distillation control is characterized by a reduction in the variability of the impurities in the products. Meeting the specification requirements on the variability of final products can make the difference between the product being a high value-added product with large market demand and being a low-valued product with a small market demand.
For customers who purchase the products produced by distillation columns as feedstock for their processes, the variability of the feedstock can directly affect the quality of the products they produce, e.g., the variability in the monomer feed to a polymerization process can directly affect the mechanical properties of the resulting polymer produced.
In addition, control performance can affect plant processing rates and utility usage. After the variability of a product has been reduced, the set point for the impurity in the product can be increased, moving the set point closer to the specification limit. If this column is the bottleneck for the process, then increasing the average impurity level, i.e., moving the impurity set point closer to the specification limit, allows greater plant processing rates.
Even if the column in question is not a bottleneck, moving the impurity set point closer to the specification limit reduces the utility usage for the column. While each of these factors can be economically important for large-scale processes, the order of economic importance is usually product quality first, followed by process throughput and finally utility reductions.
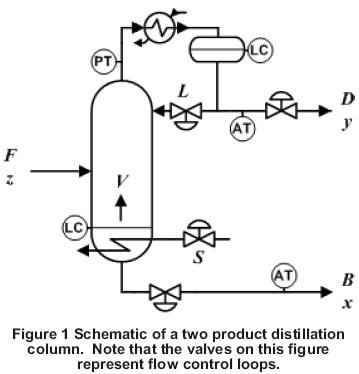
Column Schematic
A schematic of a binary distillation column with one feed and two products is shown in Figure 1:
Material balance and energy balance effects
Combining an overall steady-state material balance with the light component material balance for a binary separation yields:
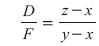
Rearranging results in:
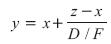
This equation indicates that as the flow rate of the distillate product, D, decreases while keeping F, z and x constant, the purity of the overhead product, y, increases. Likewise, as D increases, its purity decreases.
Because the sum of the product flows must equal the feed rate at steady state, when one product becomes more pure, the other product must get less pure. This is shown graphically in Figure 2. This is an example of the material balance effect in which the product impurity level varies directly with the flow rate of the corresponding product.
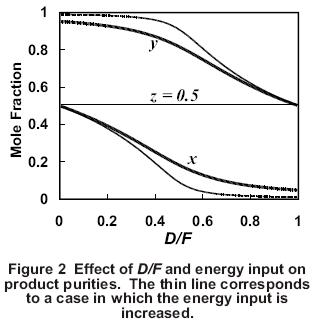
Another key factor that affects product purities is the energy input to the column, which determines the vapor rate, V, up the column. As the energy input to the column increases, the separation of the light and heavy components usually increases (Figure 2). One measure of the separation is the separation factor, S, which is given by
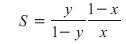
As the impurity levels in the products decrease (i.e., y®1 and/or x®0), S increases.
Another way to understand the effect of an increase in energy input to the column is to consider the vapor/liquid traffic inside the column. If V increases while D and B are kept constant, the reflux, L, increases by the same amount as V. As a result, the reflux ratio, L/D, increases. This increase in vapor/liquid traffic inside the column causes a decrease in the impurities in the products for the same D/F ratio (Figure 2). When evaluating how a column responds in a control situation, it is helpful to remember that the energy input to the column generally determines the degree of separation that the column can achieve while the material balance (i.e., D/F) determines how the separation is allocated between the two products.
Vapor and Liquid Dynamics
The difference between vapor and liquid dynamics in a distillation column can contribute to interesting composition dynamic behavior. For all but very-high-pressure columns, i.e., those operating near the critical pressure of the light key, a change in V in the reboiler can be observed in the overhead in just a few seconds while a change in the reflux flow rate requires several minutes to reach the reboiler.
The hydraulic response of a tray depends on the accumulation or depletion of liquid on it. The hydraulic time constant for flow from a tray typically ranges between 3 and 10 seconds. As a result, for a column with 50 or more trays, the overall hydraulic response time is on the order of several minutes.
As an example of the effect of the difference between liquid and vapor dynamics, consider the overhead product purity for an increase in V for a column in which the accumulator level sets the reflux flow rate and the distillate rate is held constant. Initially, the increase in vapor flow moves up the column rapidly while the liquid flow down the column remains relatively constant because the reflux rate is set by the level controller on the accumulator.
In the rectifying section, the L/V ratio determines the separating power of that section. As a result of the increase in V, the concentration of the impurity in the overhead increases initially. The increase in V begins to increase the level in the accumulator, which, after some time, leads to an increase in the reflux flow. As the increased reflux flow slowly makes its way down the rectifying section, L/V increases, causing a decrease in the impurity level in the overhead product. Therefore, for this scenario, an increase in V results in an inverse response in the concentration of the overhead product due to the difference in vapor and liquid dynamic in the column.
Entrainment
For columns operating at pressures less than about 165 psia, as V increases above 80% of flood conditions, droplets of liquid from the active area of the tray are blown in the vapor to the tray above, thus reducing the separation efficiency of the column. For certain vacuum columns, the tray efficiency can drop as much as 30% as the boilup rate increases above 80% of flood. 100% of flood corresponds to the condition for which an increase in vapor rate results in a decrease in separation for the column.
As the tray efficiency decreases because of increased entrainment, the process gain decreases, requiring larger changes in the manipulated variables to obtain the same change in the product impurity levels.
Structure Packed Columns
Columns that use sections of structured packing offer significant efficiency advantages over trayed columns for low-pressure applications because there is less pressure drop across the structured packing than across a corresponding set of trays. Because of the low liquid holdup on structured packing, these columns have faster composition dynamics than trayed columns.
The liquid holdup on the structured packing is low enough that the composition profile through the packing reaches its steady-state profile much more quickly than the reboiler and accumulator. For a column with structured packing, the dynamic lag of the accumulator and the reboiler primarily determine the dynamic response of the product compositions.
More on Distillation Control
This is the first of a series on distillation control. The next article presents the major disturbances affecting composition control and the importance of properly functioning regulatory controls.
_______
1. This material is reprinted from Chemical Process Control, 2nd Ed. with the permission of the publisher: Ferret Publishing (806 747 3872).
About the Author
Jim Riggs is a professor of chemical engineering at Texas Tech University, where he has been since 1983. He has served as an industrial consultant and presented a number of industrial short courses on various topics relating to process control. He is the author of several popular chemical engineering textbooks and co-founded the Texas Tech Process Control Consortium in 1992.
Jim Riggs
Department of Chemical Engineering
Texas Tech University
Lubbock, Texas 79410
Email: jim.riggs@ttu.edu

